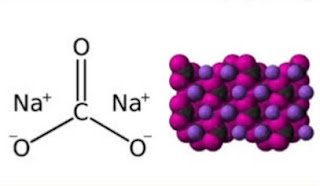
What is Washing Soda
Washing soda is the sodium carbonate and that crystallized water in which there are 10 molecules. Washing soda is made from ordinary salt. It's a solid object like a transparent crystal. This is one of the few carbonates that is water-resistant.Making prosses Of Washing Soda
1) A cool and concentrated sodium chloride reaction with ammonia and carbon dioxide to get sodium hydrogen carbonate. Sodium hydrogen carbonate is soluble in water so it becomes solid. After that, sodium hydrogen carbonate is heated by the filter and dry one hour, then gets Liquid which is known as sodaesh.NaCL+Na3+Co2+H2O=NaHCo3+NH4CI
2) Dry sodium carbonate or sodaesh is mixed with water. The making process of sodium carbonate is also called the Solvay process, but this process before calcium carbonate is heated then making carbon dioxide. This is done by washing soda.
Use Of Washing Soda
Washing soda has some of the qualities of detergent or cleanliness, due to the garbage dirt removes its Stain oil or ghee. Washing soda is also used as other cleaning agents. This can remove salts from the water. It is also used in making glass, soap, and paper. It is also used to make sodium components such as borax.
If you like this post, please share with your friends.
Thanks